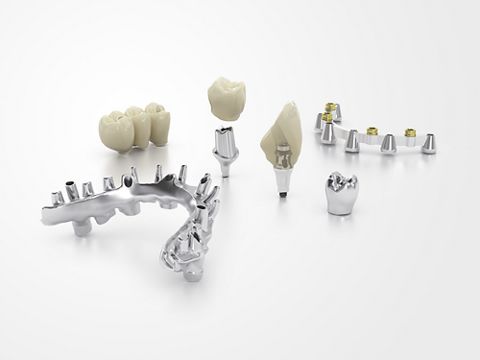
CAD/CAM materials
For all your customized prostheses
The Simeda® CAD/CAM solutions offer a wide choice of materials for all your customized prosthetics. Whether additive or 100% machined titanium, cobalt-chromium or zirconia for multiple and single implant-supported restorations, all our creations are made to meet extremely demanding production criteria.
Zirconia
- Anthogyr has more than 10 years of industrial and R&D expertise in Zirconia and offers proven durability Zirconia (> 1000 MPa) for all indications, including plural implant-supported restorations without Ti-base.
- 100% esthetic and biocompatible, zirconia is the material of choice for esthetic, implant-supported and tooth-supported restorations.
- High and consistent dynamic strength guaranteed for complex work.
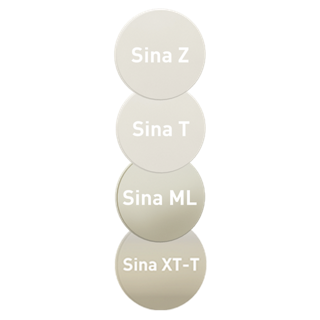
- Selection of zirconias
Sina Z: opaque zirconia, available in 7 shades
Sina T: translucent zirconia, available in 16 shades
Sina ML: multi-layer zirconia, available in 7 shades
Sina XT-T: Hybrid zirconia with gradient of translucency, available in 16 shades and 1 Bleach shade.

Medical Grade V Titanium
- Additive or 100% machined titanium depending on the type of restoration
- Durable and lightweight
- Suitable for all implant and tooth-supported restorations
- Biocompatible material specially suited to soft tissue
- Lightweight material for greater patient comfort

Cobalt Chromium
- Extremely durable
- Suitable for all implant and tooth-supported restorations
- Easy veneering for a beautiful look
- Biocompatible material
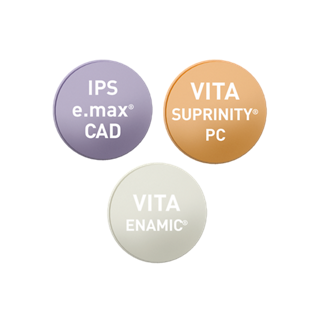
Composite Ceramics
- Large choice of materials for single, tooth-supported and anatomical restorations.
- Single-unit, crown type, inlay/onlay veneer restorations
- Beautiful materials
- Simple finishing process
- Ability to absorb masticatory force
- Range of shades available

PMMA (Polymethyl methacrylate)
Suitable for temporary prostheses
Cap and bridge restorations